【人気ダウンロード!】 percentage yield formula chemistry 291372-Percentage yield formula chemistry
ExampleUse the following formula to answer the question 2 H 2 (g) CO (g) > CH 3 OH (l) If 685kg CO is reacted with 860 kg H 2 Calculate the theoretical yield of methanol If 357 x 10^4 g CH3 OH is actually produced, what is the percent yield of methanol?The percentage yield formula is broken down into the two elements of theoretical yield and actual yield and a step by step guide through worked examples is used to visualise how these calculations should be tackled Students are given regular opportunities to test the skills which they have just learnt (or recalled) before bringing themExampleUse the following formula to answer the question 2 H 2 (g) CO (g) > CH 3 OH (l) If 685kg CO is reacted with 860 kg H 2 Calculate the theoretical yield of methanol If 357 x 10^4 g CH3 OH is actually produced, what is the percent yield of methanol?
Q Tbn And9gcrynatxciaobbvt Edbzcqmlgnhpv Acw5cqav2pwzm1fudi Ih Usqp Cau
Percentage yield formula chemistry
Percentage yield formula chemistry-This percent yield calculator helps you understand how to properly solve for percent yield using the percent yield formula Finding the percent yield using the percent yield equation or this calculator is important, especially in terms of synthetic lab work and other chemistryrelated processesConversion and its related terms yield and selectivity are important terms in chemical reaction engineering They are described as ratios of how much of a reactant has reacted (X — conversion, normally between zero and one), how much of a desired product was formed (Y — yield, normally also between zero and one) and how much desired product was formed in ratio to the undesired product(s
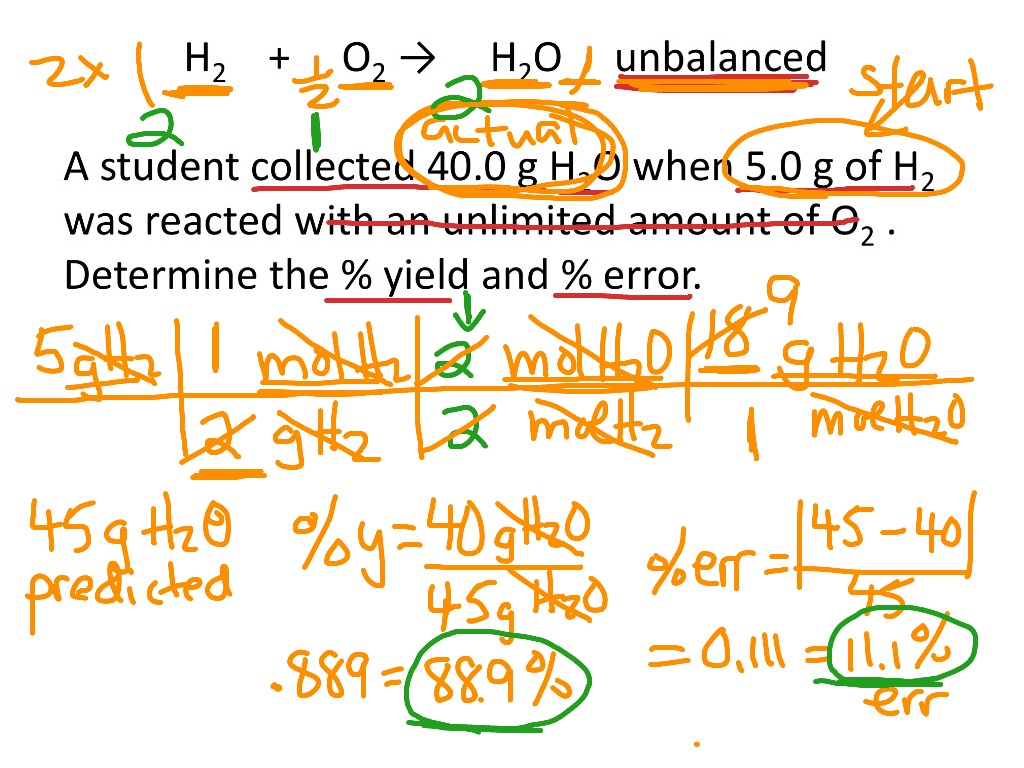


Percent Yield And Percent Error Calculations Science Chemistry Percent Yield Percent Error Showme
Theoretical Yield Formula Questions 1 Determine the theoretical yield of H 2 O (in moles) in the following reaction, if 25 moles of hydrogen peroxide are decomposed 2H 2 O 2 → 2H 2 O O 2 Answer In this reaction there is only one reactant (H 2 O 2) so it must be the limiting reactantStoichiometry will be used to determine the moles of water that can be formedPercentage yield is a concept used in chemistry which compares the theoretical yield of an experiment with the actual results observed This percent yield calculator is intended to help navigate between three key metrics percent yield, theoretical yield, and actual yieldC7H16 11O2 > 7CO2 8H2O Step One Identify the limiting reagent (the question only gives you one of the reactants, therefore, making that compound the limiting reagent) The limiting reagent is O2 Step Two Find the theoretical yield
When you look up cheem research papers, the percentage yield of each reaction is written above the arrow The example above shows a threestep method to synthesise Lacosamide, an important medication for epilepsyStep 2 is a stumbling block, with the lowest percentage yield of 37% This means that you only get 37 g of product in the lab, even though you have put in enough reactants to make 100Calculate limiting reactant and use that to calculate theoretical yieldNext, identify the decimal percentage yield using the chemical formula The actual yield of acetaminophen was reported as 0198g and is then divided by the theoretical yield of 0217g Notice that the actual yield reported was 019 less than the theoretical yield The decimal percentage of percent yield is 0
The ratio of actual yield to theoretical yield expressed in percentage is called the percentage yield \(\mathrm{percent\ yield = \dfrac{actual\ yield}{theoretical\ yield}\times100}\) Chemical reaction equations give the ideal stoichiometric relationship among reactants and products Thus, the theoretical yield can be calculated fromNext, identify the decimal percentage yield using the chemical formula The actual yield of acetaminophen was reported as 0198g and is then divided by the theoretical yield of 0217g Notice that the actual yield reported was 019 less than the theoretical yield The decimal percentage of percent yield is 0Percentage yield = \frac {Actual\;



A Level Chemistry Ocr Salters Yield Wikibooks Open Books For An Open World



Calculating Percent Yield Hey Chemistry
But the question states that the actual yield is only 3791 g of sodium sulfate With these two pieces of information, you can calculate the percent yield using the percentyield formula So, you find that 8137% is the percent yieldThe formula for percent yield is Example The medical drug aspirin is made from salicylic acid 1 mole of salicylic acid gives 1 mole of aspirin Given that the chemical formula for salicylic acid is C 7 H 6 O 3 and the chemical formula for aspirin is C 9 H 8 O 4Hydrazine, N2H4, is an oily liquid used as a rocket fuel It can be prepared in water by oxidizing ammonia with hypochlorite ions 2 NH3g ClOaq > N2H4aq Claq H2Ol When 350 g of ammonia reacted with an excess of hypochlorite ion, 252 g of hydrazine was produced What is the percentage yield of hydrazine?



How To Calculate Percent Yield In Chemistry 15 Steps



Calculating Percent Yield Hey Chemistry
Chemists have to be concerned with just how completely their reactants react to form products To compare the amount of product obtained from a reaction with the amount that should have been obtained, they use percent yield You determine percent yield of a chemical reaction with the following formula Lovely, but what is an actualPercent yield or percentage yield is the ratio of the actual yield and the theoretical yield of a chemical reaction The experimental yield is divided by the theoretical yield and multiplied by 100 to be calculated as the percent yield If the theoretical yield and the experimental yield are same then the percent yield will be 100%The formula for percent yield is Example The medical drug aspirin is made from salicylic acid 1 mole of salicylic acid gives 1 mole of aspirin Given that the chemical formula for salicylic acid is C 7 H 6 O 3 and the chemical formula for aspirin is C 9 H 8 O 4
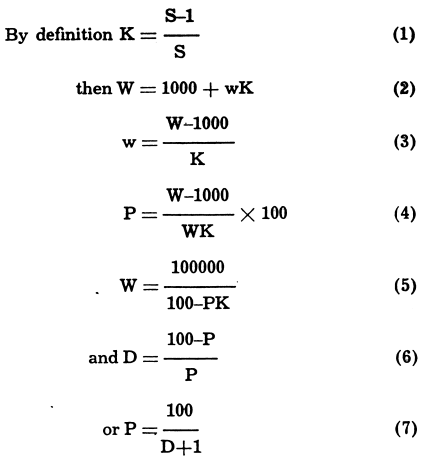


Common Basic Formulas For Mineral Processing Calculations
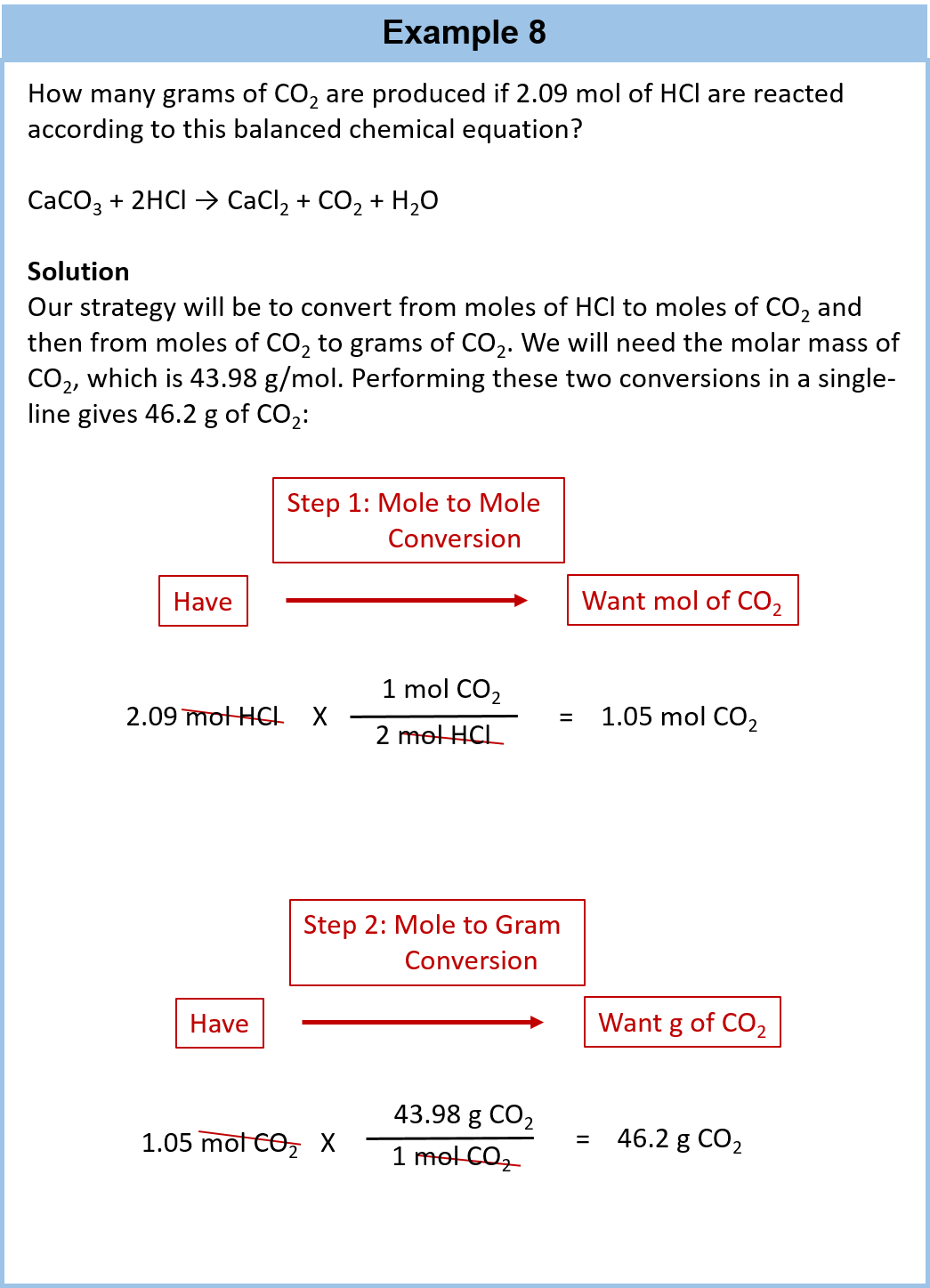


Ch104 Chapter 6 Quantities In Chemical Reactions Chemistry
Percent yield or percentage yield is the ratio of the actual yield and the theoretical yield of a chemical reaction The experimental yield is divided by the theoretical yield and multiplied by 100 to be calculated as the percent yield If the theoretical yield and the experimental yield are same then the percent yield will be 100%Percent yield or percentage yield is the ratio of the actual yield and the theoretical yield of a chemical reaction The experimental yield is divided by the theoretical yield and multiplied by 100 to be calculated as the percent yield If the theoretical yield and the experimental yield are same then the percent yield will be 100%The quantity of product that is actually produced through the reaction is called the actual yield Percentage yield can be calculated by $$\text{% yield} = \frac{\text{Actual yield}}{\text{Theoretical yield}} \times 100 \%$$ Percentage yield cannot be more than 100% as the actual yield is always less than the theoretical yield Example
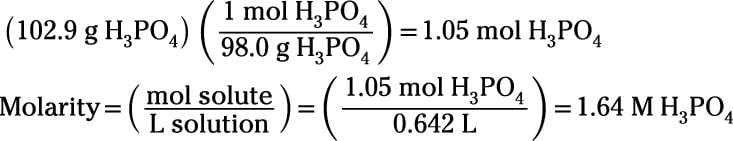


How To Measure Concentration Using Molarity And Percent Solution Dummies



Pin By Jesus Gomez On Estequiometria Chemistry Class Science Chemistry Worksheets
How to calculate the percent yield?Yield calculations are common in chemistry Calculating percent yield actually involves a series of short calculations Follow this stepbystep guide and you will be able to calculate limiting reagent, theoretical yield, and percent yield 1 Write a balanced equation for the reactionSo, all you need to put the values into the percent yield formula The percent yield equation is percent yield = (actual yield/theoretical yield) x 100% percent yield = (15 g MgO/19 g MgO) x 100% = 79 % So, the percent yield of magnesium oxide = 79 % Frequently Ask Questions What is the actual yield of a reaction?


Calculating Percentage Yield



Question Video Calculating The Percentage Yield Of The Recreation Of Aqueous Copper Sulfate With Zinc Metal Nagwa
But the question states that the actual yield is only 3791 g of sodium sulfate With these two pieces of information, you can calculate the percent yield using the percentyield formula So, you find that 8137% is the percent yieldThe formula for calculating the percent yield is Percentage yield = mass of actual yield ÷ mass of theoretical yield × 100% Let's assume that you obtained an actual yield of 850 grams Then, the percent yield would be Percentage yield of NaCl = 850 grams ÷ 993 grams × 100% Percentage yield of NaCl = 8559% Since the value ofOrganic Chemistry FriedelCrafts Alkylation of Dimethoxybenzene Description & Background 0027 mol salicylic acid X g = 372g mol 3 Divide the number of grams of product obtained experimentally, by the number of grams obtained in the theoretical yield calculations and multiply by 100 to calculate the percent yield



Yield Calculations Faculty Staff Sites



Percent Yield Chemistry Video Clutch Prep
Learn how to identify the limiting reactant in a chemical reaction and use this information to calculate the theoretical and percent yields for the reaction If you're seeing this message, it means we're having trouble loading external resources on our websitePercentage yield The percentage yield of a chemical reaction is an important consideration in industrial chemistry It can be calculated to compare the yield (quantity) of product actuallyPercentage Yield = ( Yield Obtained / Theoretical Yield ) x 100 Example In an experiment to displace copper from copper sulfate, 65 g of Zinc was added to an excess of copper (II) sulfate solution The copper was filtered off, washed and dried The mass of copper obtained was 48 g Calculate the percentage yield of copper


Percent Yield Calculator



How To Calculate Percentage Yield In Chemistry How To Wiki
To use this formula for percent yield, you need to make sure that your actual yield and theoretical yield are in the same units If the actual yield is in grams, then theoretical yield also needsThe percentage yield is calculated using this formula \Percentage~yield = \frac{yield~obtained}{theoretical~yield} \times 100\ For example, if the predicted yield is g but the actual yieldWhen it comes to chemistry, calculating the percent yield of a reaction is fairly straight forward


18 Percentage Yield



How To Calculate Percent Yield Math Wonderhowto
How to calculate the percent yield?Percentage Formula In simple terms, percent means per hundred Whenever you have to express the number between one and zero then percentage formula is needed Usually, the percentage is defined as the fraction of 100 The symbol for percentage is "%" and the major application of percentage is to compare or find the ratios Here,Yield}\times 100% Percentage yield = \frac {06} {14}\times 100% Percentage yield = 429% The percentage yield of this reaction is 429%, Scientist tries to choose reactions with a high percentage yield or high atom economy



Yield Calculations Faculty Staff Sites



Chapter 3 Section 7
The theoretical yield is a term used in chemistry to describe the maximum amount of product that you expect a chemical reaction could create You need to begin with a balanced chemical equation and define the limiting reactant When you measure the amount of that reactant that you will be using, you can calculate the amount of productPercent yield = purified percent yield = amount of P (g) theoretical yield (g) •100 Percent yield (if stoichiometry is 11) = amount of P (mol) amount of LR (mol) •100 Complicated balanced equations are uncommon in organic chemistry Many organic reactions have a stoichiometry of 11 in the balanced equation1) For the balanced equation shown below, if the reaction of 192 grams of O2 produced 676 grams of H2O, what is the percent yield?


Q Tbn And9gcrynatxciaobbvt Edbzcqmlgnhpv Acw5cqav2pwzm1fudi Ih Usqp Cau
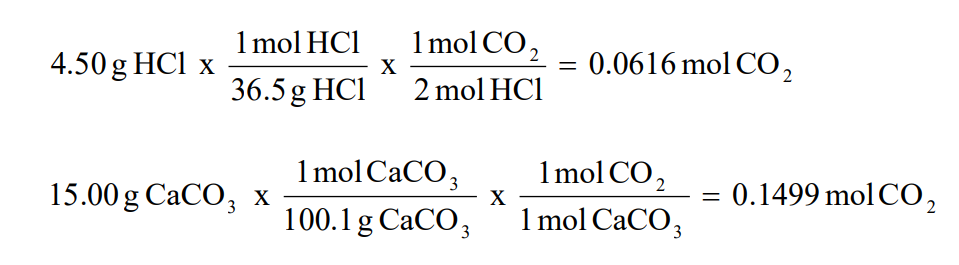


Molecular Formulas And Nomenclature
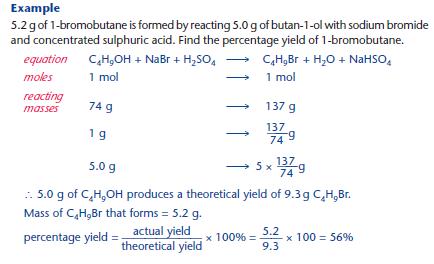
Calculate limiting reactant and use that to calculate theoretical yieldWorked Example of Percentage Yield Calculations Calculating Mass of Product from Yield Question Ammonia can be produced from hydrogen gas and nitrogen gas according to the equation below N 2(g) 3H 2(g) ⇋ 2NH 3(g) Calculate the mass of ammonia produced if 168 g of nitrogen gas produces a yield of 45%Before performing chemical reactions, it is helpful to know how much product will be produced with given quantities of reactants This is known as the theoretical yieldThis is a strategy to use when calculating the theoretical yield of a chemical reaction



Howto How To Find Percent Yield Without Actual Yield


Q Tbn And9gcqsieeu0 Yiv08aomieykfqpxaxi62zyrza1fa2mst1gqjkdvie Usqp Cau
To express the efficiency of a reaction, you can calculate the percent yield using this formula %yield = (actual yield/theoretical yield) x 100 A percent yield of 90% means the reaction was 90% efficient, and 10% of the materials were wasted (they failed to react, or their products were not captured)Percent Yield Definition A percent yield, also referred to as a percentage yield, is the ratio of the actual yield of mass of a chemical reaction, to the theoretical yield that the reaction should produce How to calculate percent yield?3 How to determine the percent yield of the reaction considering the limiting reactant Determine the percent yield of the reaction when 770 g of CO 2 are formed from burning 0 moles of C 5 H 12 in 400 moles of O 2 C 5 H 12 8 O 2 → 5 CO 2 6 H 2 O


Functional Groups Chemistry A Level Revision
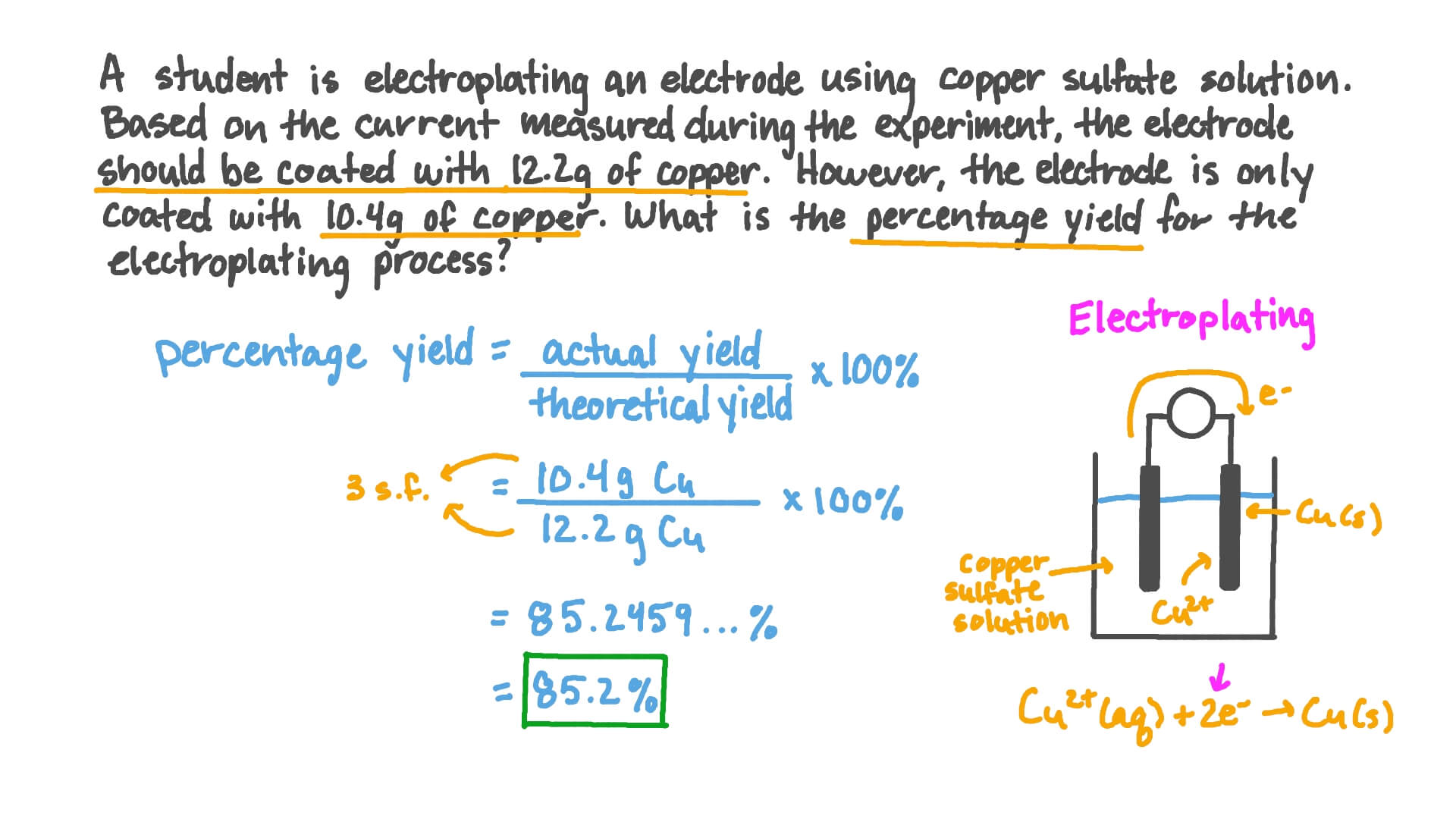


Question Video Calculating The Percentage Yield From Given Actual Yield And Theoretical Yield Nagwa
However, it is impossible for a ration to give 100 percent yield Because there is always some loss of reactant due to different factors Formula You can also find the theoretical yield yourself using the theoretical yield formula if percentage yield or actual yield is known Theoretical yield = Actual yield 100 Here is the methodPercent Yield Formula The equation for percent yield is percent yield = (actual yield/theoretical yield) x 100% Where actual yield is the amount of product obtained from a chemical reaction theoretical yield is the amount of product obtained from the stoichiometric or balanced equation, using the limiting reactant to determine product3 How to determine the percent yield of the reaction considering the limiting reactant Determine the percent yield of the reaction when 770 g of CO 2 are formed from burning 0 moles of C 5 H 12 in 400 moles of O 2 C 5 H 12 8 O 2 → 5 CO 2 6 H 2 O



Quantitative Chemistry Secondary Science 4 All
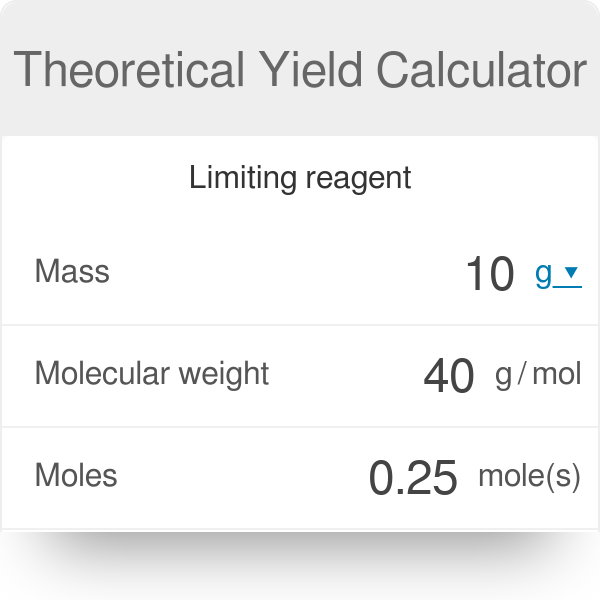


Theoretical Yield Calculator
Percent recovery computes the percentage of an original substance that is recovered after a chemical reaction is completed These mainly include purification reactions It also determines their efficiency This ScienceStruck article explains how to calculate the percent recovery of any purified substance142a Percentage yield of the product of a reaction Even though no atoms are gained or lost in a chemical reaction (law of conservation of mass), unfortunately it is not always possible to obtain the calculated amount of a product (ie 100% yield) because the reaction may not go to completion because it may be reversible or some of the product may be lost when it is separated from theSo, ideally, 336 grams of CaO should have been produced in this reaction This is the theoretical yield However, the problem tells us that only 15 grams were produced 15 grams is the actual yield It is now a simple matter to find percent yield



How To Calculate Percent Yield 3 Ways To Solve Chemistry Problems Tripboba Com
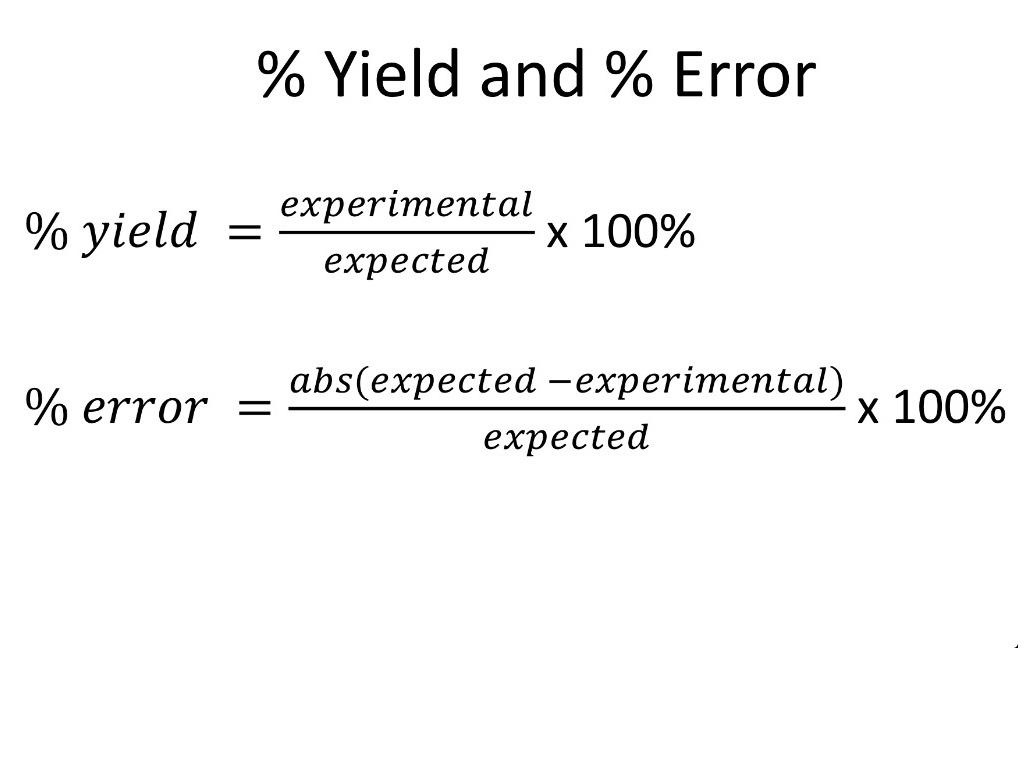


Percent Yield And Percent Error Calculations Science Chemistry Percent Yield Percent Error Showme
The formula for calculating the percent yield is Percentage yield = mass of actual yield ÷ mass of theoretical yield × 100% Let's assume that you obtained an actual yield of 850 grams Then, the percent yield would be Percentage yield of NaCl = 850 grams ÷ 993 grams × 100% Percentage yield of NaCl = 8559% Since the value of



Limiting Reactant And Reaction Yields Article Khan Academy



How To S Wiki How To Find Percentage Yield In Chemistry



Calculating Percent Recovery Percent Yield



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



Calculating Radical Yields Organic Chemistry Video Clutch Prep


The Stoichiometry Of Product Formation And Percent Yield
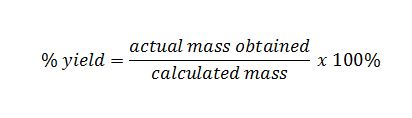


Moles Percentage Purity And Yield John Vagabond S Physics And Chemistry Blog



Reaction Yield An Overview Sciencedirect Topics



Jobian Rijo Percentage Yield Doc Chemistry Journal 5 06 Percent Yield Driving Question How Do Chemists Use Percentages And Stoichiometry To Understand Course Hero
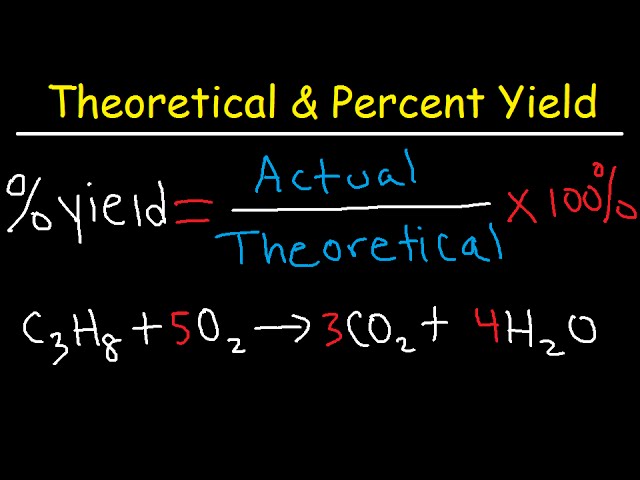


How To Calculate Theoretical Yield And Percent Yield Youtube
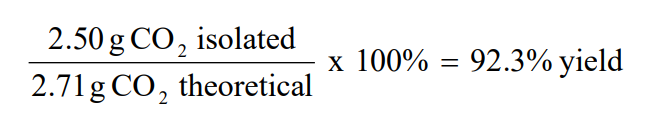


Molecular Formulas And Nomenclature



Calculating Actual Yield Given The Percent Yield Youtube



Percent Yield Percent Purity Solutions Examples Videos


Solved Calculate The Theoretical Yield And Percent Yield Chegg Com



Percent Yield Chemistry Video Clutch Prep
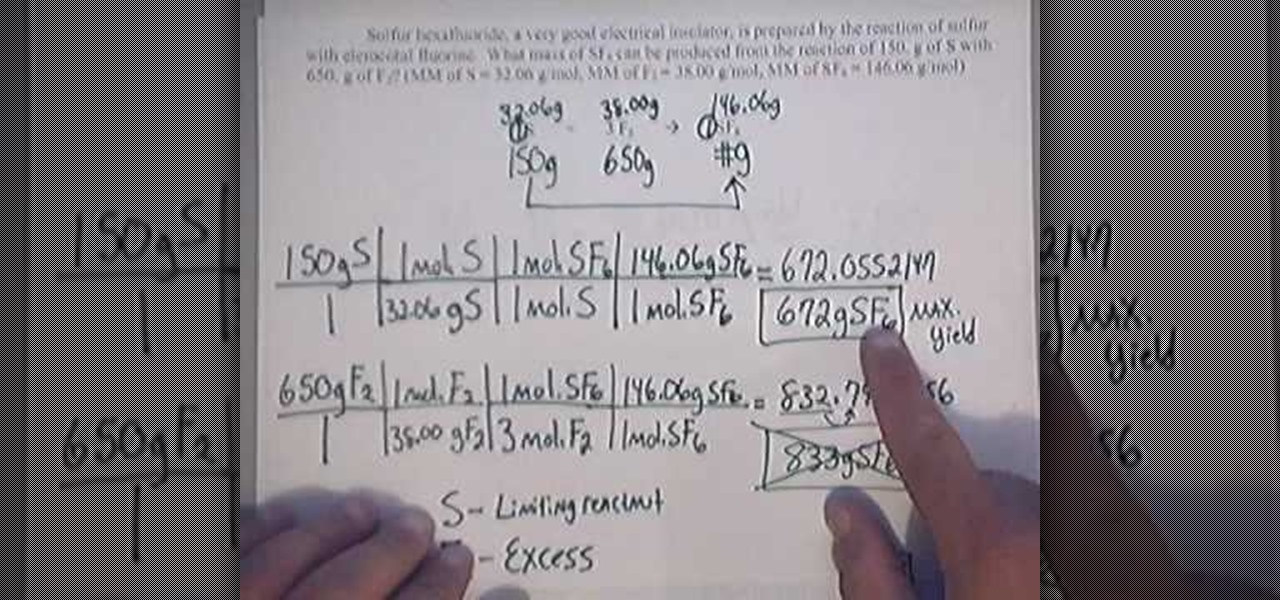


How To Calculate Percent Yield Math Wonderhowto



Reaction Percent Yield Introduction And Practice Exercises
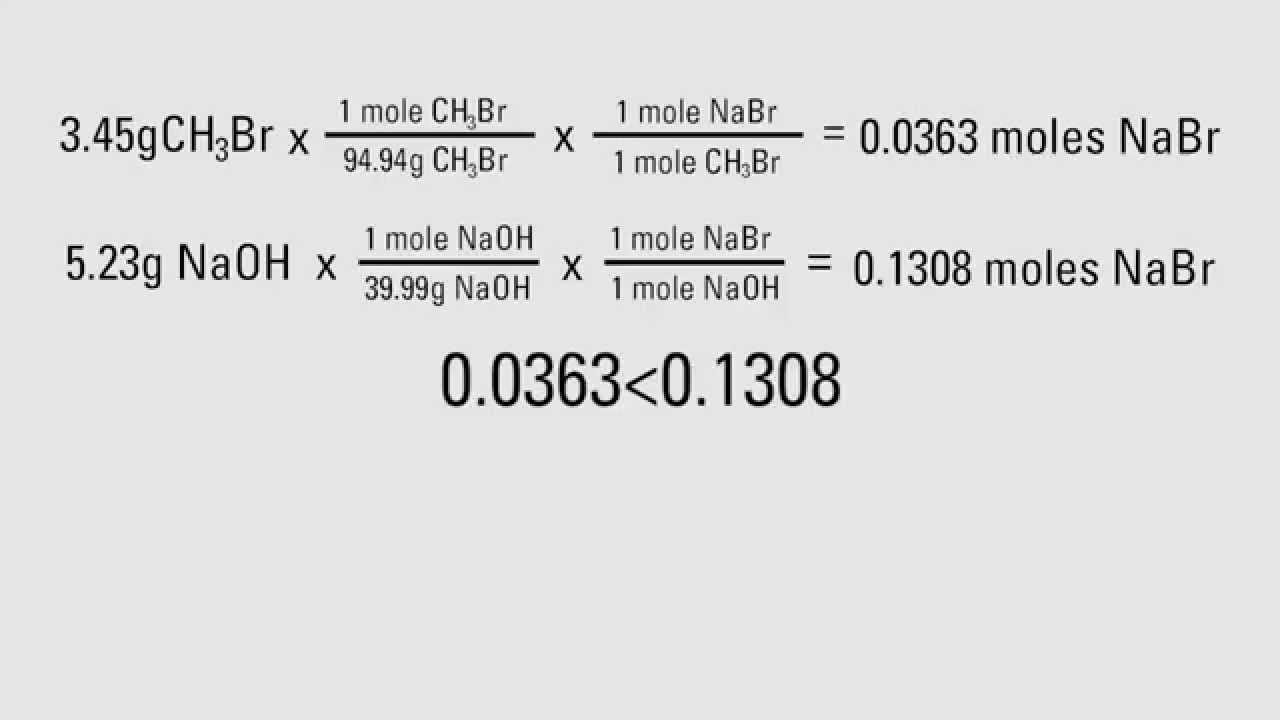


How To Calculate Theoretical Yields Youtube



Yields Introductory Chemistry



Percentage Yield Lab Answers Schoolworkhelper


Q Tbn And9gcrjyqnm Hsuw1zecxl5b8r5elmfwvpiet 7gqpwpfgze5a6h7ox Usqp Cau



How To Calculate Theoretical Yield 12 Steps With Pictures



Chapter 1 The Mole And Stoichiometry Ppt Download
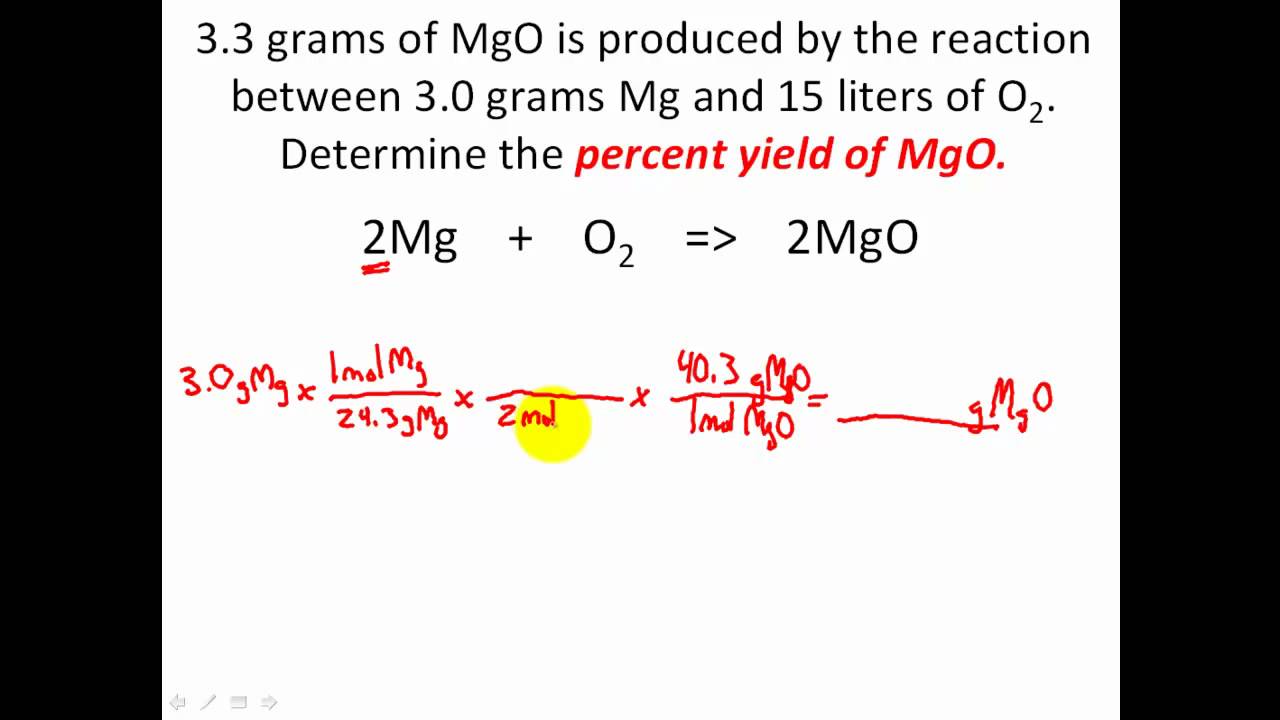


Stoichiometry Solving Percent Yield Stoichiometry Problems Youtube


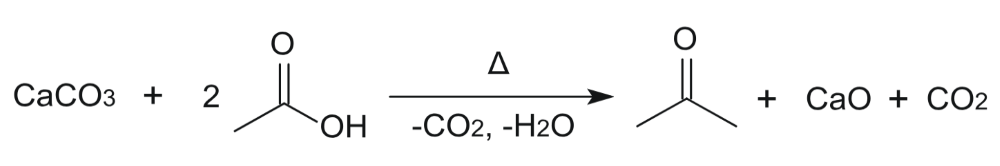
3



Percent Yield Tutorial Explained Practice Problems Crash Chemistry Academy Youtube


Calculation Of Theoretical Yield Organic Chemistry I 212 01



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


8 6 Limiting Reactant Theoretical Yield And Percent Yield From Initial Masses Of Reactants Chemistry Libretexts
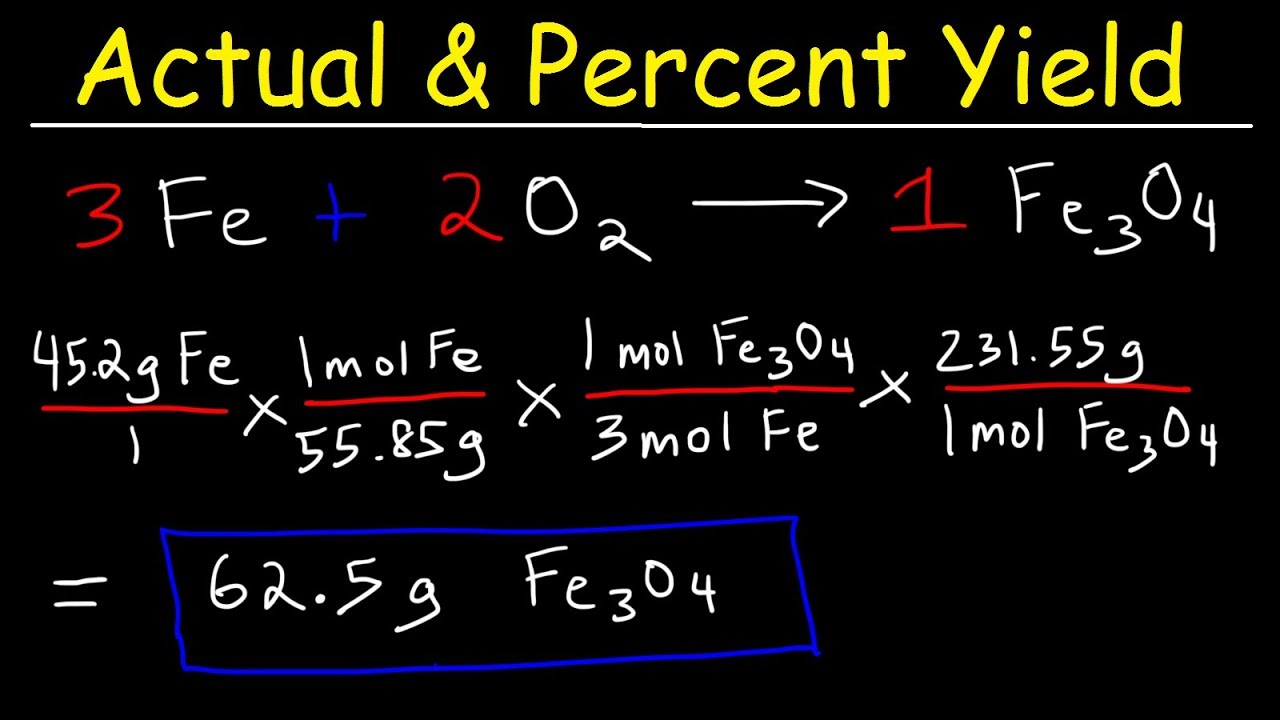


How To Calculate The Percent Yield And Theoretical Yield Youtube
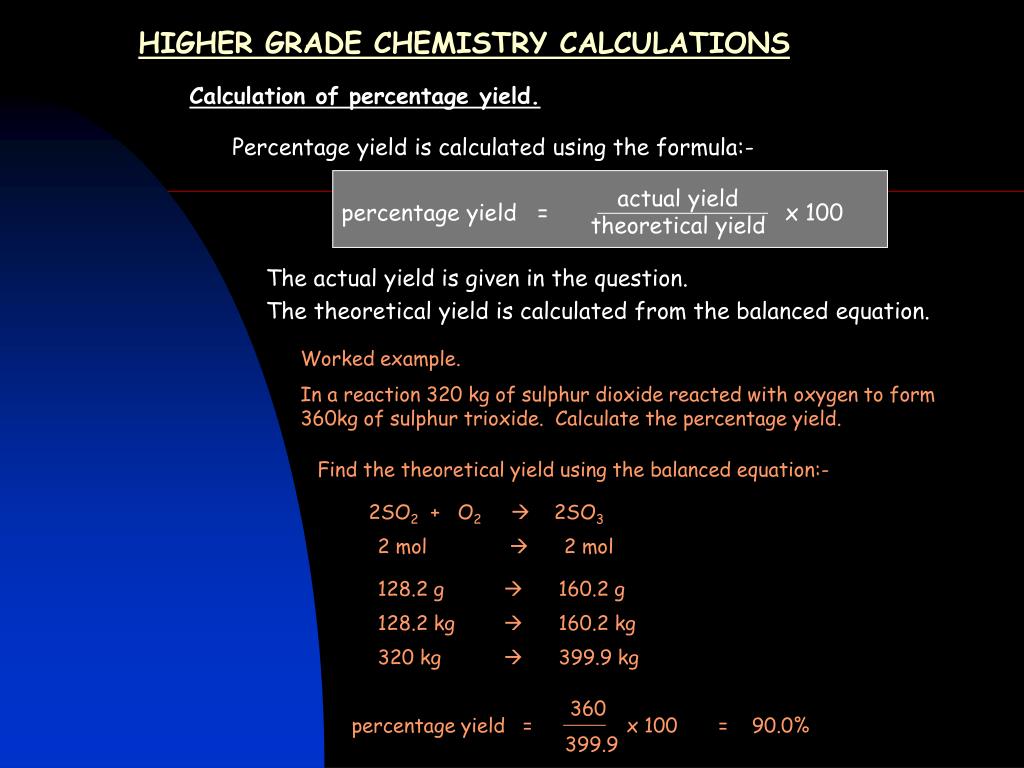


Ppt Higher Grade Chemistry Calculations Powerpoint Presentation Free Download Id



Percentage Yield Lab Answers Schoolworkhelper
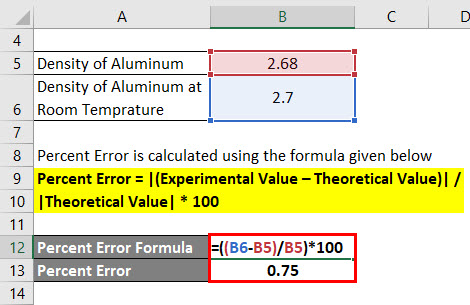


Percent Error Formula Calculator Excel Template



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com
/148302528-56a12f323df78cf77268383a.jpg)


Percent Yield Definition And Formula
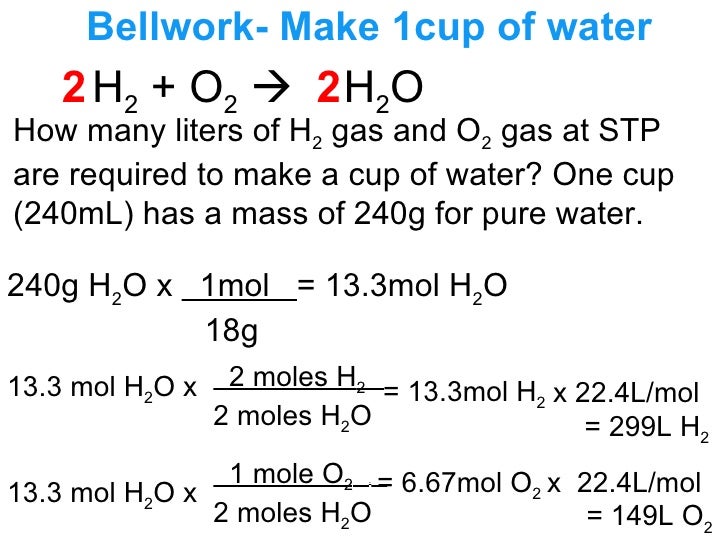


Lecture 12 3 Limiting Reagents And Percent Yield


Chemistry Atom Economy And Percentage Yield


Www Assignmentexpert Com Homework Answers Chemistry Answer Pdf
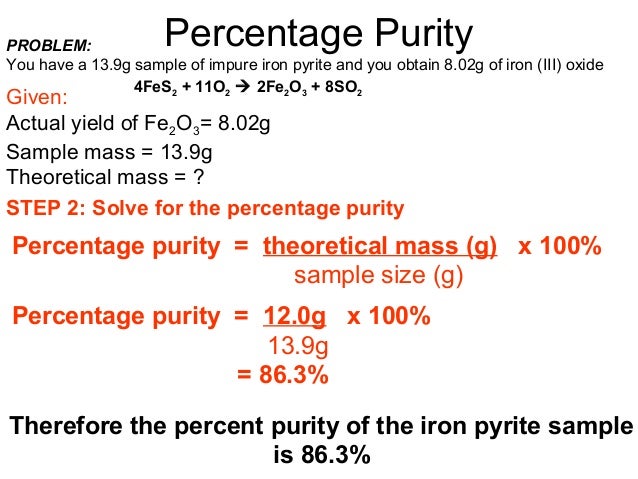


18 Percentage Yield



Theoretical Actual And Percent Yield Problems Chemistry Tutorial Youtube
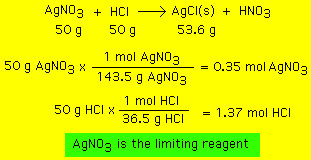


Quantitative Chemistry Theoretical And Percent Yield



Quantitative Chemistry Secondary Science 4 All



Howto How To Find Percentage Yield In Chemistry



Yields Introductory Chemistry



Calculating Percent Yield Hey Chemistry



Reaction Yield Protocol
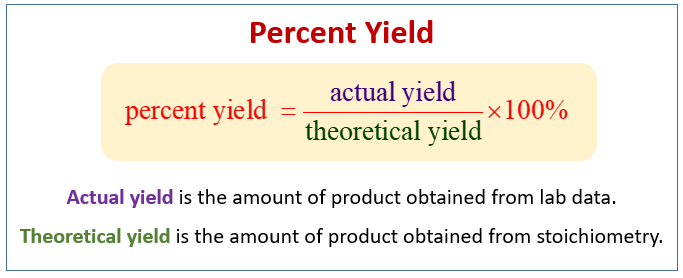


Stoichiometry And Percent Yield Examples Solutions Worksheets Videos Games Activities



Synthesis Of Benzil From Benzoin Labmonk
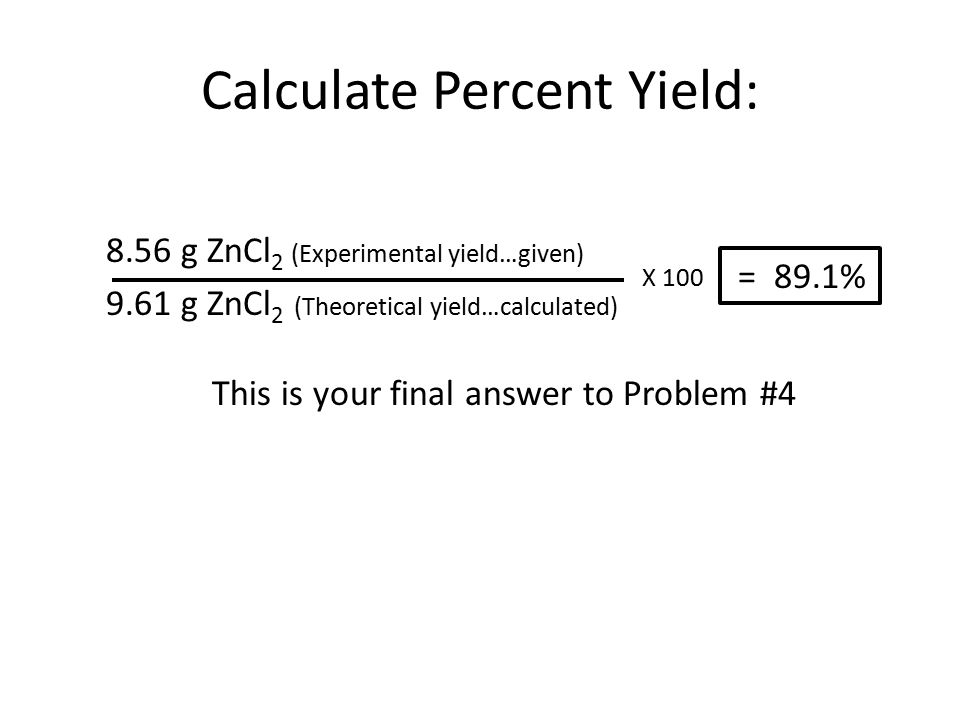


Unit 8 Percent Yield Calculations Ppt Download



2 Calculate The Theoretical And Percent Vields Of Your Reaction Showing Your Calculate Step By Step Homeworklib



Percent Yield And Percent Error Calculations Science Chemistry Percent Yield Percent Error Showme



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



Reaction Percent Yield Introduction And Practice Exercises



Percentage Yield Lab Answers Schoolworkhelper



Magnesium Oxide Percent Yield Lab Report Schoolworkhelper



How To S Wiki How To Calculate Percentage Yield



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



Percent Yield Calculator



Yield Calculations Faculty Staff Sites


Chemistry Atom Economy And Percentage Yield



Solved The Yield Of A Chemical Reaction Is Defined As The Rati Chegg Com


3 4 Percent Yield Chemistry Libretexts



C2 Chemistry Powerpoint Presentation Free Online Download Ppt Aertax


5 3 Calculating Reaction Yields Problems Chemistry Libretexts



Percent Yield Worksheet


Calculating Percentage Yield


Atom Economy Yield
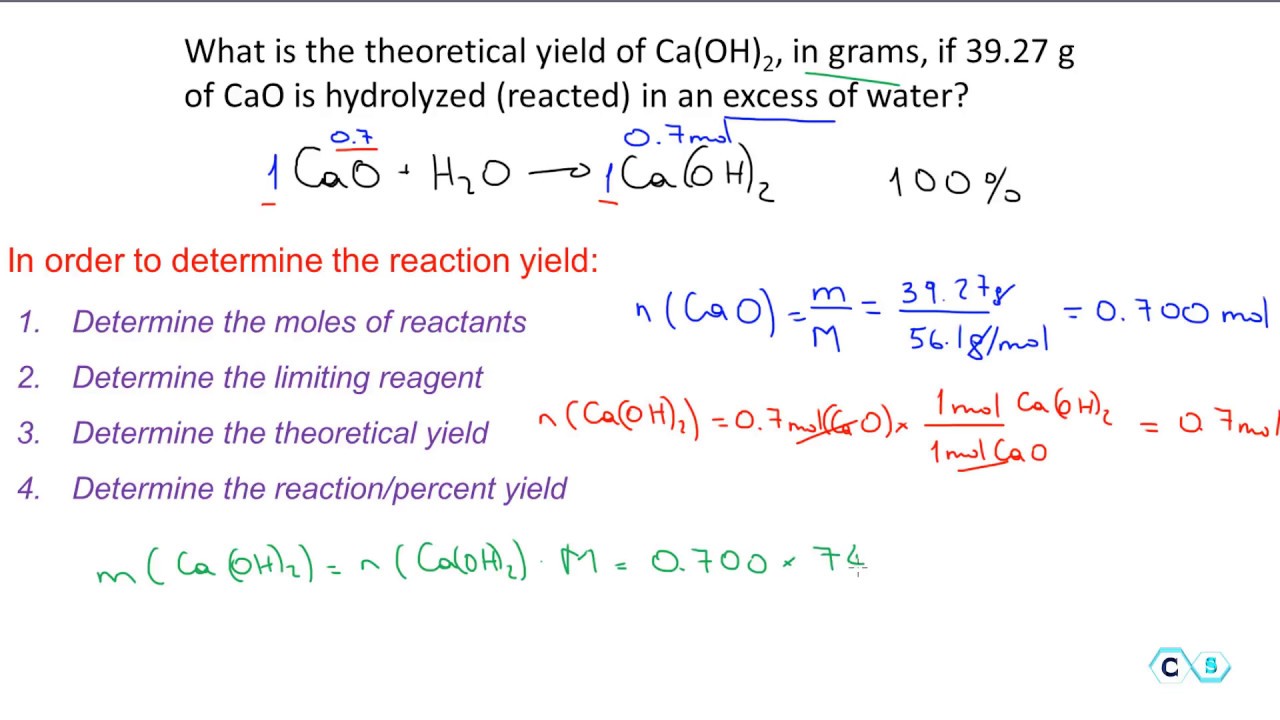


Reaction Percent Yield Introduction And Practice Exercises
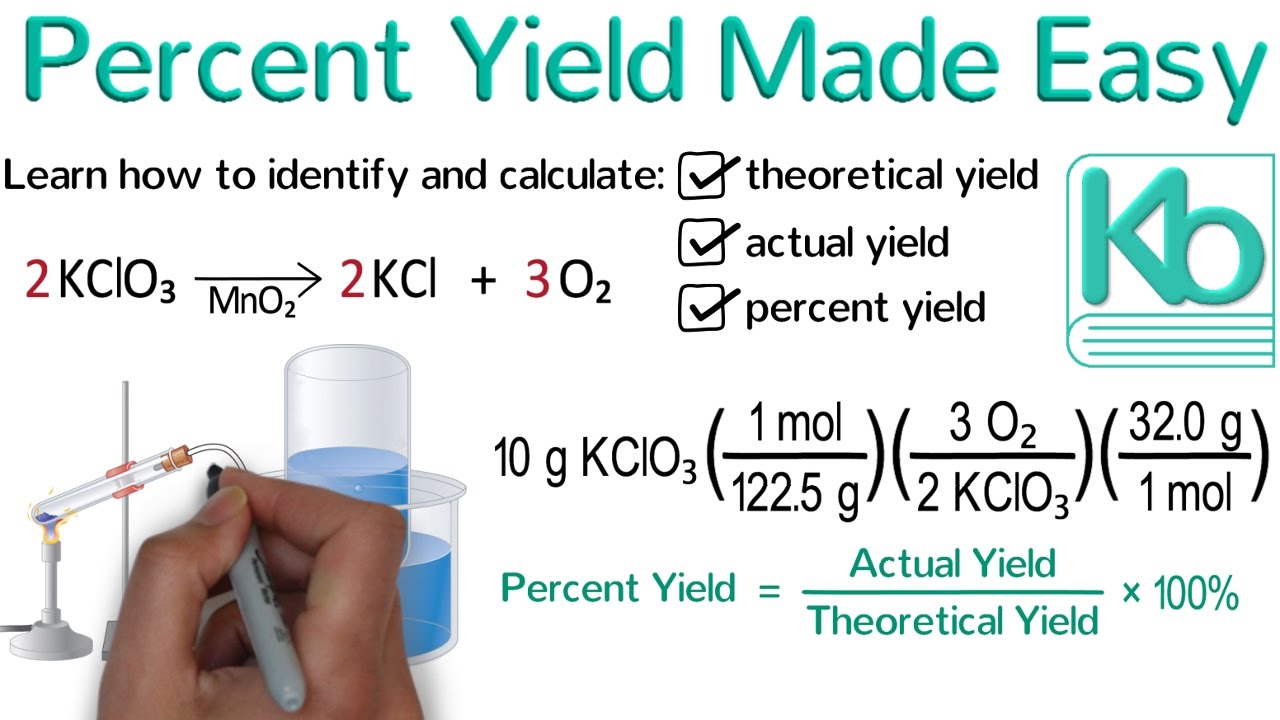


Percent Yield Made Easy Stoichiometry Tutorial Part 4 Youtube
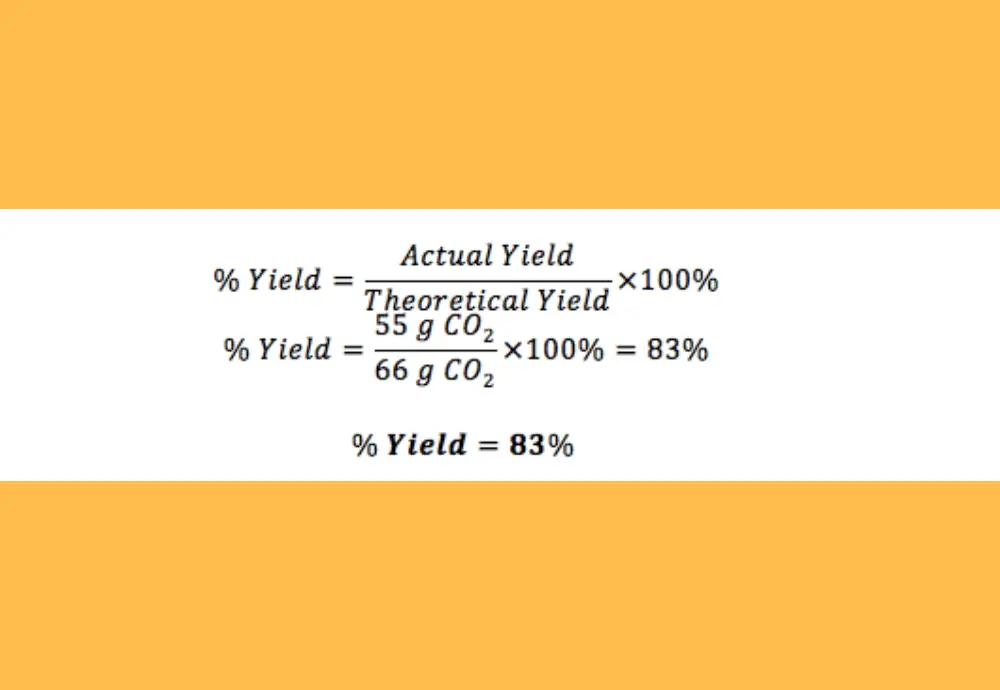


Percent Yield Calculator 100 Free Calculators Io
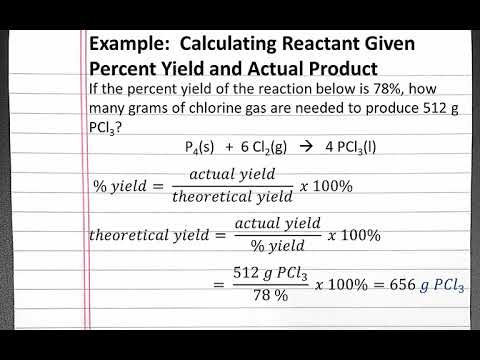


Chemistry 101 Calculating Reactant Given Percent Yield And Actual Product Youtube
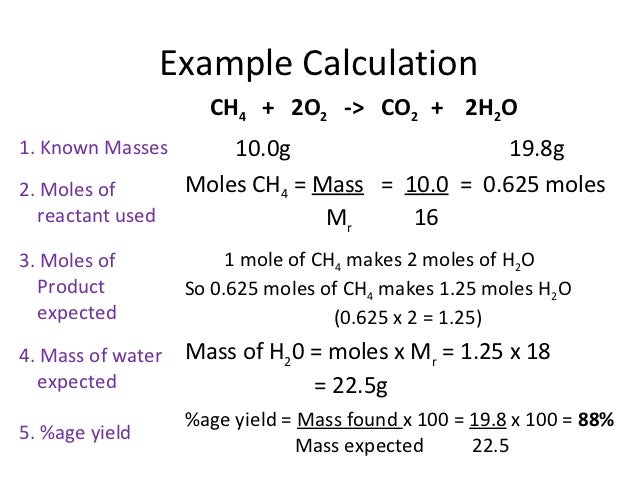


Chemistry Calculations Percent Yield And Atom Economy



A Level Chemistry Ocr Salters Yield Wikibooks Open Books For An Open World
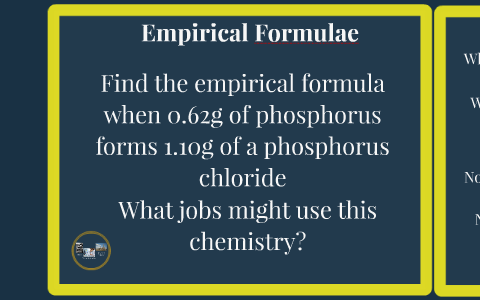


Percentage Yield By David Gabb



Reaction Yield Protocol


コメント
コメントを投稿